Notifications
DETERMINATION OF HLB NUMBER OF A SURFACTANT BY SAPONIFICATION METHOD
Aim: To determine the HLB value of a given surfactant by saponification method
Requirements:
Chemicals: Fatty acid ester ex.Glycerol monostearate (gms),0.5N alcoholic potassium hydroxide (KOH), stearic acid, ether, 0.5N hydrochloric acid, 0.1N sodium hydroxide, phenapthalene indicator.
Apparatus: Round bottom flask, reflux condenser, beakers, burette, pipette, conical flask.
Principle: The HLB value of surfactant can be determined based on saponification reaction and the formula for determination of HLB value.
HLB = 20( 1-s/a)
Where: s = Saponification number
a = Acid number
The saponification number is the number of milligrams of potassium hydroxide required to neutralize the fatty acids resulting from the complete hydrolysis of 1g of fat.
Acid number. a number expressing the acidity of a substance, equal to the number of milligrams of potassium hydroxide needed to neutralize the free fatty acids present in one gram of the substance.
Procedure:
1. Preparation of 0.5N alcoholic KOH: Dissolve around 4g of KOH in 3 to 5 ml of distilled water in a volumetric flask and make up total volume to 100 ml with alcohol. Allow it to stand for about 24 hours and separate out clear liquid by decantation. Use this clear solution for experiment. Alcoholic KOH is used, because surfactant freely soluble in alcohol than in water. The solubility improved by alcohol hydrolysis is effective one.
2. Determination of saponification number: Weigh accurately 0.5g of GMS and transfer into round bottom flask, add 15 ml of alcoholic potassium hydroxide to it and reflux on boiling water bath for about half an hour.
Reflux separately 15 ml of alcoholic potassium hydroxide (without GMS) on boiling water bath for about half an hour blamk reading.
Cool both the solutions to room temperature and titrate separately against 0.5 N hydrochloric acid using phenolphthalein as the indicator.
(End point: pink to colorless or slightly yellowish)
Let the titrate reading for sample (GMS) be V1 and blank be as V2.
3. Determination of acid number: Weigh accurately 0.5g of stearic acid, add it to a mixture of 10 ml of alcohol and 10 ml of ether. If stearic acid does not dissolved in the solvent mixture, warm it on water bath until it dissolves.
Titrate solutions of stearic acid against 0.1 N sodium hydroxide using phenolphthalein as the indicator.
Let the titrate reading be V3.
Observation:
Saponification number = __________
1. Volume of 0.5 N HCL consumed by sample (V1)=______ml
2. Volume of 0.5 N HCL consumed by blank (V2)=_______ml
Acid number=_________
1. Volume of 0.1 N sodium hydroxide consumed (V3)=____ml
Calculation:
Calculation for saponification number:
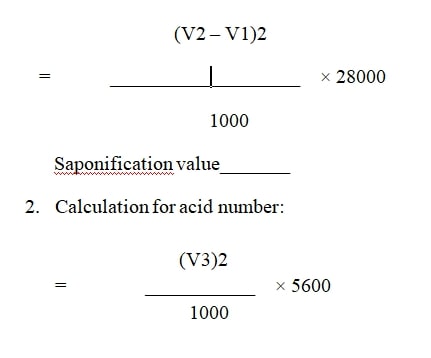
Acid number =__________
3. Calculation of HLB value:
HLB = 20( 1-s/a)
Report: The HLB value of given surfactant was found to be_______